what electron geometry is associated with sp3d hybridization?
All of them Dont for get the. Hybridization can also describe an entire set of bonds.
 |
| What Electron Group Geometry Is Associated With Sp3 Hybridization Quora |
Sp3 hybridization means that a mix of 4 completely equivalent molecular orbitals is formed from 1 s and 3 p atomic orbitals.
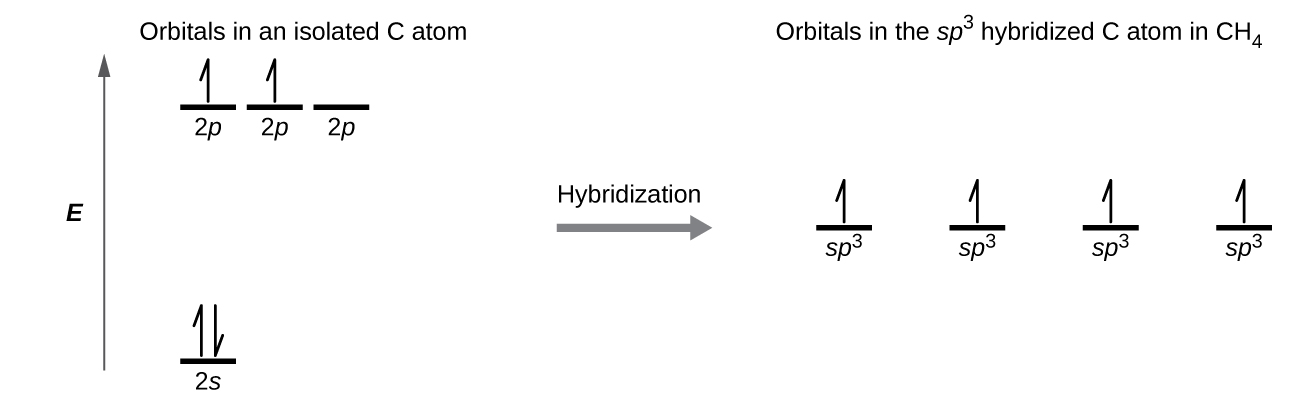
. We can classify the electron geometries according to the AXn system. Each of these sp 3 d 2. Jun 6 2015 The hybridization uses first s orbitals then p orbitals and finally d orbitals. These orbitals hybridise to form six new sp3d2 hybrid orbitals which are projected towards the six corners of a regular octahedron in SF6.
A molecule containing a central atom with sp3d hybridization has an electron. For sp3d hybridized central atoms the only possible molecular geometry is trigonal bipyramidal. These 6 orbitals are directed towards the corners of an O c t a h e d r o n. For instance in BeF 2 Be undergoes sp-hybridization which has a.
There is 1 electron pair in each of the 4 orbitals which will repell. We can describe the bonding in PCl 3 very much as we do NH 3. For nitrogen the first sp 3 orbital has 2 electrons then one electron for each of the remaining three 3. The sp 3 d hybrid orbitals have 20 s 60 p and 20 d characters.
There one s atomic orbital three p atomic orbitals and. For understanding these various types of bonds it is essential to look at the different orbitals involved in electron sharing. These six sp3d2 hybrid orbitals overlap. Since waters oxygen is sp³ hybridized the electronic geometry still looks.
PF 5 In PF 5 one of the electrons in 3s orbital is promoted to the higher 3d orbital so that it has five. 1 Answer Ernest Z. The sp3d3 hybridization has a pentagonal bipyramidal geometry ie five bonds in a plane one bond above the plane and one below it. Sp3d Class AX5 Shape trigonal bipyramidal Angle 120 in plane 90 above below Total of e-.
Now lets see how that happens by looking at. Verified by Toppr In SF 6 one electron each from 3s and 3p orbitals is promoted to 3d orbitals The six orbitals get hybridized to form six sp 3 d 2 hybrid orbitals. Sp 3 d 2 hybridization is the mixing of s p and d atomic orbitals of the same electron shell to form sp 3 d 2 hybrid orbitals. Youll get a detailed solution from a subject matter expert that helps you learn core concepts.
Sp Hybridization- When one s- and one p-orbital intermix it is called hybridization. Four sp 3-hybridized orbitals three of which are shared with electrons from other atoms and the fourth containing a. If all the bonds are in place the shape is also trigonal bipyramidal. Oxygens 6 valence electrons sit in hybridized sp³ orbitals giving us 2 paired electrons and 2 free electrons.
As it is a positive species the central atom contains 6 electrons out of which 2 e will be involved in forming two sigma bonds with two chlorine atoms and there will 2 lone pairs. 5 Total bonding directions. S p 3 d 2 hybridization has 1 s 3 p and 2 d orbitals that undergo intermixing to form 6 identical s p 3 d 2 hybrid orbitals. For carbon each sp 3 orbital has 1 electron.
If there are only four bonds. Hybridization is a theory that is used to explain certain molecular geometries that would have not been possible otherwise. D s p 3 hybridization cannot result from square pyramidal geometry because it only produces five hybrid orbitals whereas square pyramidal requires six. S p 3 d 2 is a possible.
 |
| A Schematic Description Of Sp Sp 2 And Sp 3 Hybridization In Download Scientific Diagram |
 |
| How To Determine Hybridization A Shortcut Master Organic Chemistry |
 |
| What Are The Shapes And Bond Angles Of Sp Sp2 Sp3 Sp3d Sp3d2 Hybridised Orbitals Respectively Quora |
 |
| Hybridization Of Sf4 Hybridization Of S In Sulfur Tetrafluoride |
 |
| Hybridization Of Atomic Orbitals Sigma Pi Bonds Sp Sp2 Sp3 Youtube |
Posting Komentar untuk "what electron geometry is associated with sp3d hybridization?"